 pO2 and pH2O ESTIMATION... 'o' You
can use this calculator to determine the partial pressure of oxygen (pO2) -- or any other gas species in a mixture -- from
ambient temperature, ambient pressure (in the gas phase), fractional concentration
of the gas species of interest in a dry gas mix, and the percent
saturation of water vapor (i.e., relative humidity) in the gas phase. Oxygen
(or other gases) are diluted by water vapor, and the degree of that dilution
depends on RH and temperature. pO2 and pH2O ESTIMATION... 'o' You
can use this calculator to determine the partial pressure of oxygen (pO2) -- or any other gas species in a mixture -- from
ambient temperature, ambient pressure (in the gas phase), fractional concentration
of the gas species of interest in a dry gas mix, and the percent
saturation of water vapor (i.e., relative humidity) in the gas phase. Oxygen
(or other gases) are diluted by water vapor, and the degree of that dilution
depends on RH and temperature.
In the example at right, pressure is sea level standard atmospheric pressure (760
torr), temperature is the typical mammalian body temperature (37 °C),
etc. Note that at this temperature the saturation vapor pressure of water
is about 47.6 torr (this is not affected by the total pressure in the system).
Other considerations for this calculator:
- The calculated pO2 value is applicable
for the gas phase, and also for dissolved oxygen, as long as the solution
is fully saturated with O2.
- The default pressure (torr or kilopascals, kPa) is obtained from the
current data file; the default temperature is 37 °C, and the default
fractional gas concentration is .2095 (20.95%, the normal oxygen content
of dry atmospheric air).
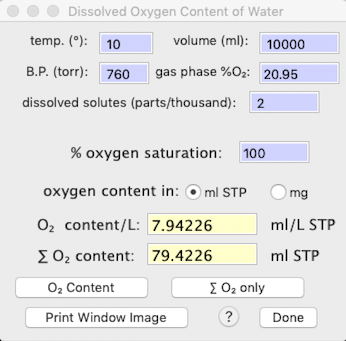
DISSOLVED OXYGEN... 'w' You
can use this calculator to determine the amount of oxygen dissolved in a given volume water, as a function of partial pressure, temperature, salinity (dissolved solutes), and other factors. Results include both the oxygen content per liter, and the total oxygen content in the specified volume of water. Output units are user-selectable as ml O2 (STP) or mg O2.
The default salinity value (2 parts/thousand) is reasonable for freshwater. For seawater, use a value of 35 parts/thousand, and for typical physiological saline, use 9 parts/thousand.
In the example at right, pressure is sea level standard atmospheric pressure (760
torr), temperature 10 °C, the water is fully saturated with oxygen,
and there are 2 parts/thousand of dissolved solutes (reasonable for fairly fresh water). The calculator provides the dissolved oxygen per unit volume, and for the total volume.
|

